Abstract
Introduction: Blinatumomab is a bispecific antibody approved for the use in adults with relapsed acute lymphoblastic leukemia (ALL) but only about 50% of patients respond. A few brief reports have suggested that differences in T cell subsets between responders and non-responders may contribute to the failure of blinatumomab, however our understanding of the immune microenvironment in ALL is still in its infancy. We hypothesize response may be related to an immunosuppressive T cell microenvironment and in this project sought to define this microenvironment using both phenotypic and functional assays.
Methods: Peripheral blood or bone marrow aspirate samples were obtained from patients with newly diagnosed and relapsed/refractory ALL as well as healthy donors. Mononuclear cells (consisting of lymphocytes and leukemic cells) were isolated via ficoll hypaque gradient. Immunophenotyping was performed by staining samples with a panel of 33 lanthanide isotope labeled antibodies and analyzing via CyTOF (Table 1). In parallel, functional assays were performed by labeling mononuclear cells with Cell Trace Violet and incubating on plates coated with an anti-CD3 antibody for 5 days. T cell division was assessed by flow cytometry. A portion of samples were cultured with blinatumomab 10 ng for 5 days and T cell division and the number of CD19+ cells were enumerated. Limited phenotyping and expression of checkpoint molecules after stimulation with CD3 or blinatumomab was assessed using multi-parameter flow cytometry for the following cell surface markers: CD45, CD3, CD4, CD8, CD56, CD19; and the checkpoint molecules PD-1, PL-L1, PD-L2, TIGIT, TIM-3, and CTLA4.
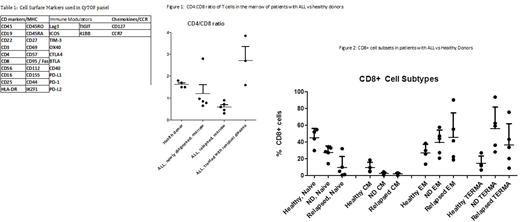
Results: Bone marrow aspirate from 15 patients and PBMC from 4 patients with ALL were collected from patients with de novo (9), relapsed (7) or treatment refractory ALL (3). Bone marrow aspirate from 4 healthy donors were obtained as controls. The CD4:CD8 ratio in the healthy patient samples averaged 1.65. and was similar but more varied in the newly diagnosed patient samples. There was a marked expansion of CD8+ cells in the relapsed patient samples, while in the patients who had been treated but had residual disease, there was a marked reduction in CD8+ cells (figure 1). There was a trend to a higher average number of T regs in the patient samples than in the controls (6.02 vs 9.1). Within the CD8 T cell population, there was a trend toward higher numbers of effector memory (EM) and terminally differentiated T cells (TERMA) in the patient samples compared to the healthy donors (figure 2). CD57 was upregulated in the patient samples, and expressed primarily on the EM and TEMRA CD8+ cells. Expression of immune modulators on T cells was variable across the patient samples, with PD-1, TIGIT, and ICOS being the most highly expressed. Commonly expressed immune modulators on the leukemic blasts included LAG-3, CD112, 41BB and PD-L2. In terms of functional status, T cells from the patient samples did not proliferate in any of the 10 samples tested. This is in contrast to healthy donors where T cells divide in all instances. Six of 8 patient samples showed vigorous T cell division in response to culture with blinatumomab, with a marked reduction in CD19+ cells noted in the responders. Following blinatumomab stimulation, T cells demonstrated upregulation of PD-1 and TIGIT, while no changes were observed in the expression of TIM-3, CTLA-4 or PD-L1/2.
Conclusion: These results highlight the differences in the T cell subsets and function between healthy donors and patients with acute lymphoblastic leukemia. ALL tends to lead to an expansion of CD8+ cells within the bone marrow, with a higher proportion of EM and TEMRA CD8+ cells as compared to healthy controls. High expression of CD57 was found on the EM and TERMA CD8+ T cell subsets of patients; while this is a well known marker of senescence it may also represent T cell exhaustion in this population. The most commonly upregulated checkpoints included TIGIT and PD-1 on T cells as well as the TIGIT ligand, CD112, on the tumor, suggesting that these axes may be targetable in ALL. The lack of response to CD3 stimulation in the patient samples suggests that the microenvironment in ALL is highly immunosuppressive. At this time patient numbers are small, however these results provide the beginning of a functional and phenotypic description of T cell subsets in the marrow and periphery of patients with ALL.
Leonard:Amgen: Research Funding. Druker:Aptose Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Third Coast Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Cepheid: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aileron Therapeutics: Consultancy; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Oregon Health & Science University: Patents & Royalties; MolecularMD: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; GRAIL: Consultancy, Membership on an entity's Board of Directors or advisory committees; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees; ALLCRON: Consultancy, Membership on an entity's Board of Directors or advisory committees; Patient True Talk: Consultancy; Millipore: Patents & Royalties; ARIAD: Research Funding; Henry Stewart Talks: Patents & Royalties; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Monojul: Consultancy; Novartis Pharmaceuticals: Research Funding; Bristol-Meyers Squibb: Research Funding; Fred Hutchinson Cancer Research Center: Research Funding; Beta Cat: Membership on an entity's Board of Directors or advisory committees; McGraw Hill: Patents & Royalties. Tyner:Gilead: Research Funding; Incyte: Research Funding; Aptose: Research Funding; Janssen: Research Funding; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees; Array: Research Funding; Takeda: Research Funding; Constellation: Research Funding; Genentech: Research Funding; AstraZeneca: Research Funding. Lind:Fluidigm: Honoraria; Amgen: Research Funding; Celgene: Research Funding; Janssen Pharmaceutical R&D: Research Funding; Monojul: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal